01/11/2022
The US Food and Drug Administration (FDA) released a draft guidance, “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions” late December 2021. The draft guidance provides a framework for assessing if software modeling used to support medical device premarket submissions are credible. Regulatory submissions often lack adequate explanation of the rationale for the models’ credibility for the context of use (COU). The COU is the specific role and scope of the model used to address the “question of interest.”
Steps of the framework are to:
“Describe the question(s) of interest to be addressed in the regulatory submission that will be informed by the computational model.
Define the context of use (COU) of the computational model.
Determine the model risk.
Identify and categorize the credibility evidence, either previously generated or planned, which supports credibility of the computational model for the COU.
Define credibility factors for the proposed credibility evidence and set prospective credibility goals for each credibility factor, with a plan to achieve these goals.
Perform prospective adequacy assessment: if the credibility goals are achieved, will the credibility evidence be sufficient to support using the model for the COU given the risk assessment?
Generate the credibility evidence by executing the proposed study(ies) and/or analyzing previously generated data.
Determine if credibility goals were met and perform post-study adequacy assessment: does the credibility evidence support using the model for the COU given the risk assessment?
Prepare a report on the credibility of the computational modeling and simulation (CM&S) for inclusion in the regulatory submission.”
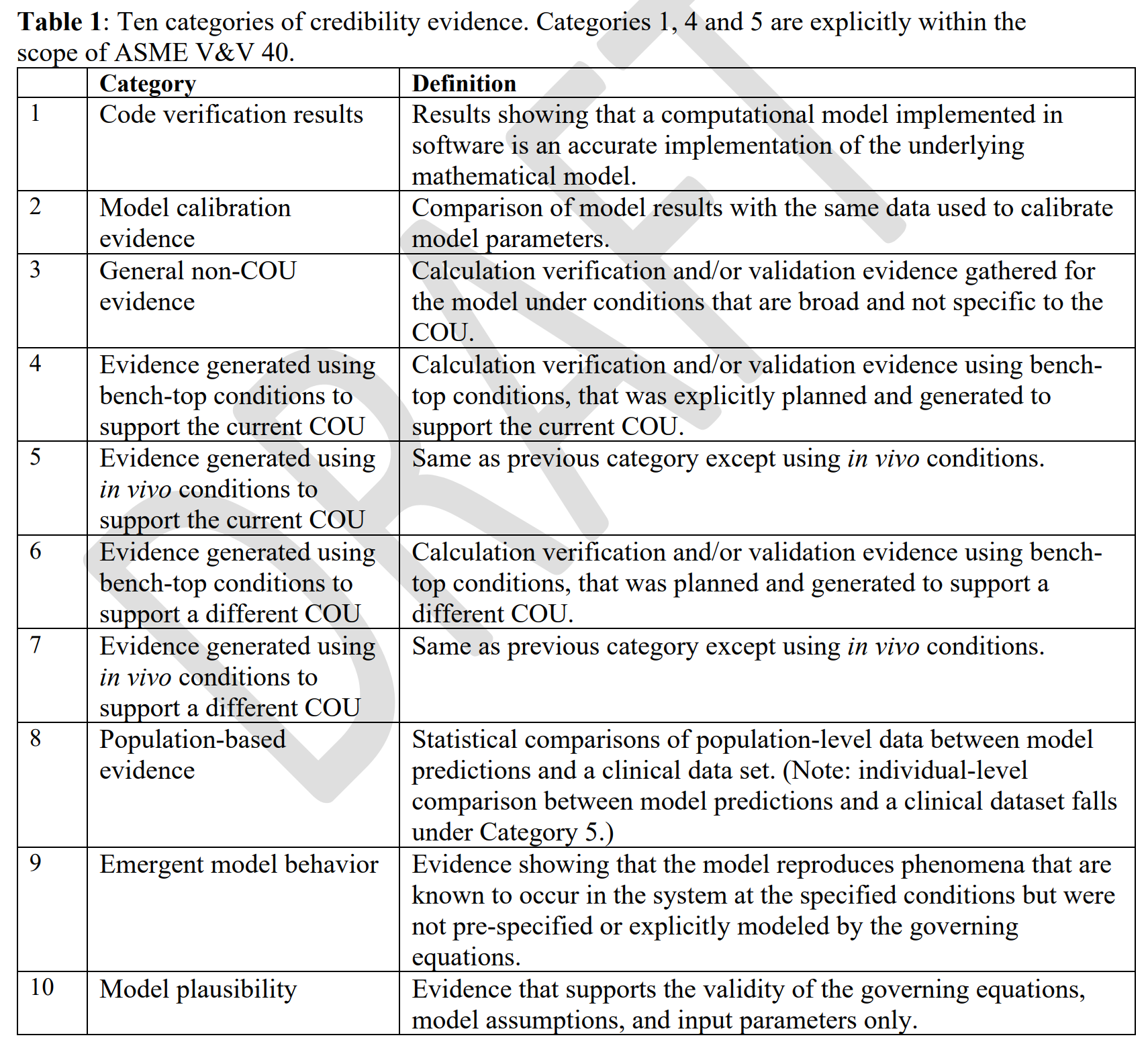
Credibility evidence refers broadly to evidence that supports the credibility of a model since evidence could be previously generated or planned and may not come from traditional verification and validation methods. There are ten categories of credibility evidence.
CM&S can be used in a variety of ways to support a regulatory submission. These are:
In Silico Device Testing, which are medical devices that are simulated using models that generate safety and/or effectiveness data. These can be used with simulations of subjects to test performance of simulated devices or actual devices (e.g., simulated subject data sent back to the device).
CM&S used within medical device software, which includes software as a medical device (SaMD), for medical use independent of hardware, or software in a medical device (SiMD), which is used as part of a device.
In Silico Clinical Trials, using simulated subjects with modeled variability (e.g., anatomical differences), can be used independently or along with real world clinical trials.
CM&S-based qualified tools can be submitted to the FDA as a proposal for consideration as a non-clinical model of safety, effectiveness, or performance.
The use of CM&S is increasing, and the draft guidance, when finalized, supports the evidence needed to increase confidence in model credibility used to support regulatory submissions. Utilizing credible modeling data can reduce burden and shorten the timeline before submissions.
Submit comments through February 22 HERE.
You may also enjoy our related blogs:
- The Clinical Pathways Team
Enjoy this blog? Please like, comment, and share with your contacts.